Urgent Drug Recall - Teva Octreotide for Injectable Suspension
Urgent Drug Recall
Teva Octreotide for Injectable Suspension
(DIN 02503751, 02503778, 02503786)
Toronto, Canada (January 10, 2026) - Teva Canada Limited is conducting a voluntary Type I recall of Teva Octreotide for injectable suspension (10 mg, 20 mg, and 30 mg per vial, sustained-release; DIN 02503751, 02503778, and 02503786) for potential quality concerns due to good manufacturing practice (GMP) issues identified at a third-party site. To date, Teva Canada Limited has not received any reports of complaints or adverse events related to the lots being recalled.
Products Subject to the Recall
The affected products are as follows:
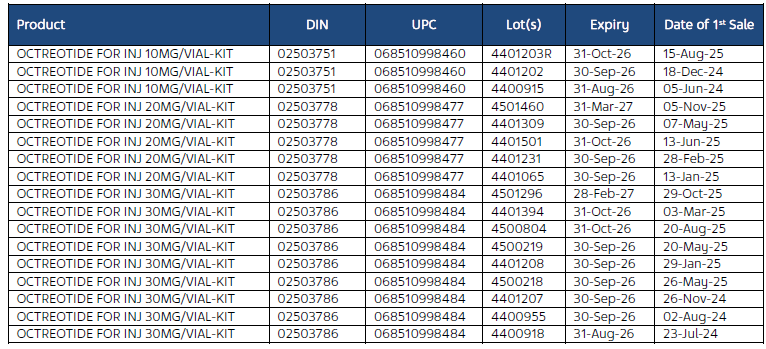
The Product
• Prescription injectable drug supplied as a single-dose kit for intramuscular use.
Kit Contents:
- One single-dose vial containing prescribed dose of octreotide (as octreotide acetate) in a lyophilized powder form for suspension.
- One prefilled syringe with diluent (usually about 2 mL) to reconstitute the powder.
- One vial adapter to help transfer diluent into the drug vial.
- One sterile injection needle (often 1.5″, 19-gauge safety needle).
- Instruction booklet for preparation and administration.
What to Do
Consumers who have purchased Teva Octreotide are asked to check the lot number on the product label. If it has the lot number listed above, you are advised to return it to the pharmacy/store where you purchased it and obtain an alternative or replacement product.
Teva Canada Limited is notifying its distributors, retailers, and health care professionals to arrange for the return / credit of any returned recalled product lots. Distributors and retailers that have inventory of the recalled product have been advised to immediately stop distribution and isolate remaining quantities in their control and return the recalled product lots to Teva Canada Limited.
Medical Questions or Side Effects
This Public Communication is not intended as medical advice. To understand the implications of this information to your health, it is important that you consult a doctor or health care professional. Managing marketed health product-related side effects depends on healthcare professionals and consumers reporting them. Any case of unexpected side effects in patients using Teva Octreotide should be reported to Teva Canada Limited and/or Health Canada.
Contacts
Teva Canada Limited
Medical Affairs & Drug Information
30 Novopharm Court
Toronto, Ontario M1B 2K9
1-800-268-4127, Option 3
druginfo@tevacanada.com
Media Inquiries
Public Affairs Office
public.affairs@tevacanada.com
Health Canada
Suspected adverse reactions may be reported to Health Canada through the Canada Vigilance Program:
Toll-free: 1-866-234-2345
Online: www.healthcanada.gc.ca/medeffect
Fax: 1-866-678-6789
Teva Canada Limited
Teva is committed to improving health by providing innovative treatments and quality generic and biosimilar medicines. We are a global company that serves over 200 million patients each day, in Canada that translates to over 175,000 prescriptions a day filled with a Teva product. For 60 years, Teva Canada has been part of the fabric of Canadian families by providing accessible and innovative healthcare solutions including vital patient support programs. For more information visit: www.tevacanada.com.